Molecule and their types
Molecule
A molecule is the smallest particle in a chemical element or compound that has the chemical properties of that element or compound.
There are many types of molecules. Molecules are classified on different bases.
- Classification on the base of numbers of atoms
On the basis of the number of atoms present in the molecule, it has the following types.
Monoatomic:
If only one atom is present in a molecule then it is known as monoatomic.
Example: He, C, Na
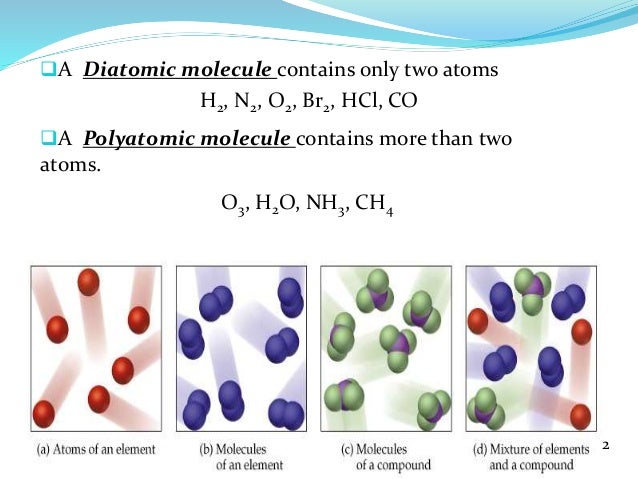
Polyatomic
If more than one atom is present in molecules then it is known as polyatomic. It has further types like.
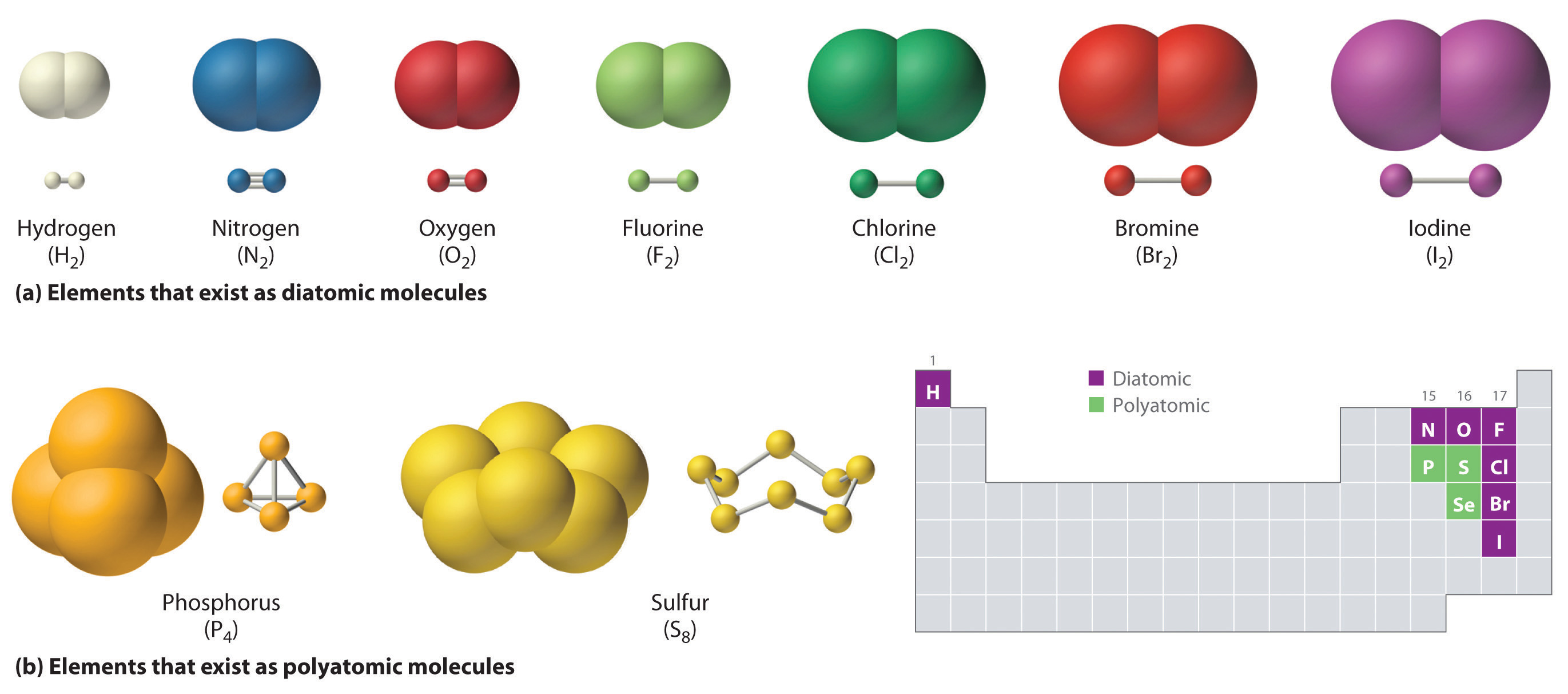
Diatomic:
If two atoms are present, it is known as a diatomic Example. H2 O2 Cl2, HCl.
Triatomic
If three atoms are present in a molecule then it is known as a triatomic example
H2O, Na2O.
2. Classification on the basis of types of atom
On the basis of types of atoms, molecules have two types
Heteroatomic
If different atoms are present in a molecule then it is known as heteroatomic molecules.
Example: HCl, NaOH.
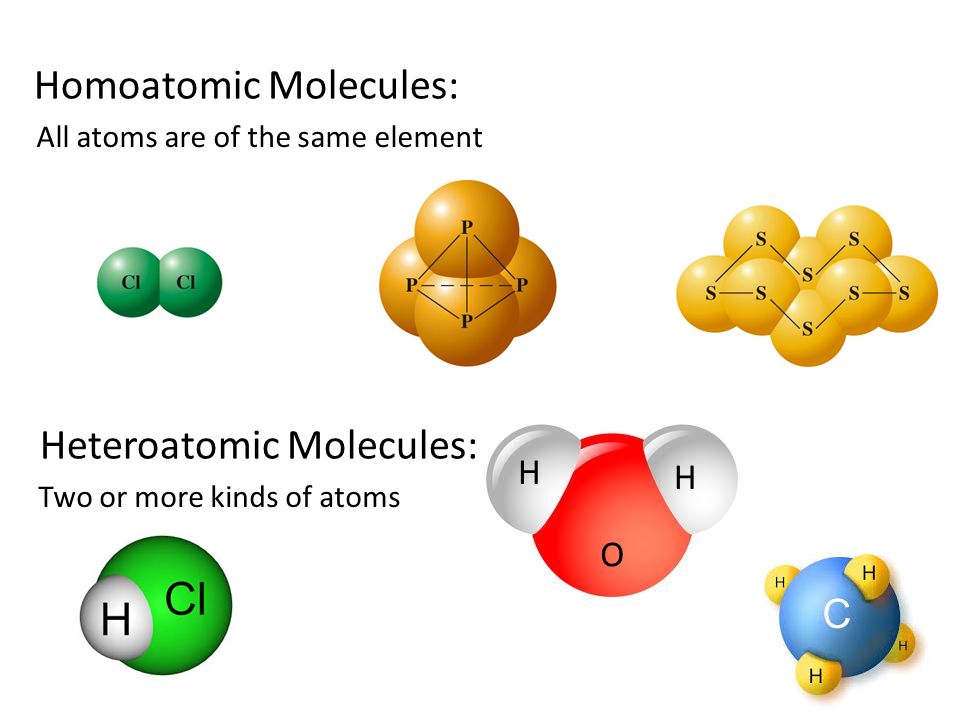
Homoatomic
If the same atoms are present in a molecule then it is known as the homoatomic molecule
Example. H2, O2
3 Classification on the basis of molecular mass
on the basis of molecular mass, molecules have two types
Macromolecules:
If molecules have larger weight then it is known as macromolecules.
Examples: Nucleic acids, Protein, Hemoglobin, etc.
Micromolecules
If molecules have smaller weight then it is known as micromolecule.
Example: water, ammonia, etc.
4. Classification on the basis of electronegativity difference
There are two types of molecules on the basis of electronegativity difference between the bonded atoms.
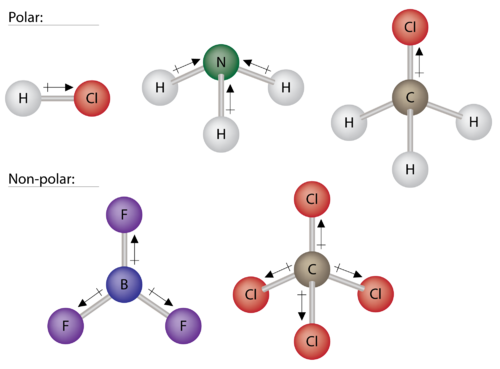
Polar molecules
If the electronegativity difference between the two bonding atoms is below 1.7 and above 1, then this molecule has polar nature.
Example: HCl
Non-polar molecules
If the electronegativity difference between the bonding atoms is below 1, then this molecule has nonpolar nature. Example: CH4, C2H5









0 Comments
Thanks for visiting blog. if you have any query please let me know.