Different rules are used for the
nomenclature of alcohol and alkyl halide. Every organic compound has some
common names which are used locally in different areas.
Nomenclature of alkyl halide
` When one of the halogens ( F,
Cl, Br, I) is attached with alkyl then this class is known as monohaloakanes.
Monohaloalkanes are also called alkyl halides.
Common names of alkyl
halide
Alkyl halides are named
on the basis of the nature of the alkyl group. First, we write the name of the alkyl group
then we will the name of halogens.
For example:
IUPAC names of alkyl
halides
Alkyl halides have the following
IUPAC rules.
1. Select the longest continuous carbon chain from the whole molecule which has a functional group
 |
| nomenclature of alkyl halides |
2. Number the carbon chain
from that side where the functional group (F, Cl, Br, I) gets the lowest possible
number.
 |
| nomenclature of alkyl halides |
3. If the same alkyl
substituent presents more than one time then use the prefix di, tri, and so on
before the name of the alkyl group.
 |
| alkyl halide nomenclature, |
4. The position of each substituent is represented by an appropriate number and separated by commas. If a different substituent is present on the same carbon atom then we will repeat this
number to represent each substituent.
 |
| alkyl halide nomenclature |
Nomenclature of alcohols
There are two systems for naming alcohols.
Common names of alcohols
Alcohols are generally known by their
common names, which are obtained by adding the word alcohol after the names of
alkyl group.
For example:
IUPAC rules for naming alcohol
Following rules are used for naming
alcohols.
1. First select the
longest carbon atom chain which contains the hydroxyl group as the parent name.
The names alcohol ends with –ol.
 |
| alcohol nomenclature |
2. Number the carbon atom
chain from that side where group (OH) is present. The position of the hydroxyl
group is indicated by the number placed before the parent name.
3. If more than one –OH
groups are present on the carbon atom chain then we use suffix diol, triol, etc.
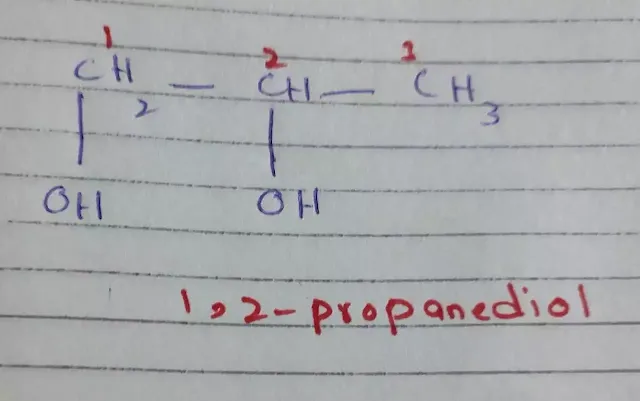 |
| alcohol nomenclature, |
4. If a double bond is also
present in the molecule along with
-OH group then we
numbered the carbon atom chain in such a way that hydroxyl groups get the lowest
possible number rather than unsaturation.
5. If a higher priority
functional groups (carboxyl acid, aldehyde, and ketone) are present in a molecule
along with the hydroxyl group then we named that compound according to priority
groups, and –OH group is represented as a substituent in these compounds. –OH is named as hydroxyl prefix in the form of
substitution.
 |
| alcohol nomenclature |









0 Comments
Thanks for visiting blog. if you have any query please let me know.