- Spectroscopy is the study of matter and its interaction with electromagnetic radiation.
- All matter contains molecules; these molecules have bonds that are continually vibrating and moving around.
- These bonds can vibrate with stretch motions or bend motions.All bonded atoms molecularly vibrate unless they are at zero degrees Kelvin.
- IR radiation does not have enough energy to induce electronic transitions as seen with UV.
- Absorption of IR is restricted to compounds with small energy differences in the possible vibrational and rotational states.
- The interactions of infrared radiation with the matter may be understood in terms of changes in molecular dipoles associated with vibrations and rotations.
- There are several types of vibrations that cause absorptions in the infrared region
- Vibrational transitions in a molecule occur between distinct vibrational energy levels. Intramolecular vibrations and intermolecular vibrations are contributing to the spectrum.
- Infrared (IR) spectroscopy: based on IR absorption by molecules as undergo vibrational and rotational transitions.
For a molecule to absorb IR, the vibrations or rotations within a molecule must cause a net change in the dipole moment of the molecule. The alternating electrical field of the radiation (remember that electromagnetic radiation consists of an oscillating electric field and an oscillating magnetic field, perpendicular to each other) interacts with fluctuations in the dipole moment of the molecule. If the frequency of the radiation matches the vibrational frequency of the molecule then radiation will be absorbed, causing a change in the amplitude of molecular vibration.
Fundamental vibrations (Absorptional frequencies)
A molecule has as many degrees of
freedom as the total degree of freedom (x, y, z ) of its individual atoms. Each atom has
three degrees of freedom (corresponding to the Cartesian coordinates), thus in
an N-atom molecule there will be a 3N degree of freedom.
In molecules, movements of the
atoms are constrained by interactions through chemical bonds.
Translation
- the movement of the entire
molecule while the positions of the atoms relative to each other remain fixed: 3
degrees of translational freedom.
Rotational transitions – interatomic distances remain constant but the entire molecule
rotates with respect to three mutually perpendicular axes: 3
rotational freedom (nonlinear), 2 rotational freedom (linear).
Vibrations –
relative positions of the atoms change while the average position and
orientation of the molecule remains fixed.
Degree of freedom linear non-linear
Translational 3 3
Rotational 3 2
Vibrational 3N-5 3N-6
total 3N 3N
N= no of atoms in a molecule
IR is also known as vibrational spectroscopy ...so here vibrational transition occurs.
Molecular rotations
Rotational transitions are of little use to the spectroscopist. Rotational levels are quantized, and absorption of IR by gases yields line spectra. However, in liquids or solids, these lines broaden into a continuum due to molecular collisions and other interactions.
Molecular vibrations
The positions of atoms in a molecule are not fixed; they are subject to a number of different vibrations. Vibrations fall into the two main categories of stretching and bending.
Vibrations can either involve a
change in bond length (stretching) or bond angle (bending).To be accompanied by IR absorption a stretch or bend must change
the dipole moment of the molecule
Molecules with symmetric bonds such as N2, O2, or F2 do not
absorb in the infrared since bond stretching does not change
the dipole moment of the molecule.
Vibrations
Any change in the shape of the molecule- stretching of bonds, bending of
bonds, or internal rotation around single bonds
Stretching vibrations
- Imagine two balls attached by a spring, representing a diatomic molecule. The movement of each ball toward or away from the other ball along the line of the spring represents a stretching vibration
- Change in the inter-atomic distance along the bond axis
- Occurs at higher energy: 4000-1250 cm-1.
- Stretching modes are typical of higher energy than bending modes
- If the bond is compressed, there is a restoring force that pushes the atoms apart, back to the equilibrium bond length. Similarly, if the bond is stretched, there is a restoring force that forces the atoms back closer together, again restoring the equilibrium bond length.
- The frequency of the stretching vibration depends on two factors:
(1) The mass of the atoms
(2) The stiffness of the bond
Heavier atoms vibrate more slowly than lighter ones, so a C-D bond will
vibrate at a lower frequency than a C-H bond.
Stronger bonds are stiffer than weaker bonds, and therefore require more
force to stretch or compress them.
Thus, stronger bonds generally vibrate faster than weaker bonds.
So O-H bonds which are stronger than C-H bonds vibrate at higher
frequencies.
- The energy of the stretch decreases as the mass of the atoms is increased
C-H 3000 cm
-1
C-C 1200 cm
-1
C-O 1100 cm
-1
C-Cl 750 cm
-1
C-I 500 cm
-1
- The energy of the stretch is related to the hybridization in the order sp > sp2 > sp3
C-H sp 3300 cm-1
C-H sp
2 3100 cm-1
C-H sp
3 2900 cm-1
- Stretching modes are often divided into two symmetric and asymmetric stretch; the asymmetric stretch is usually of higher energy.
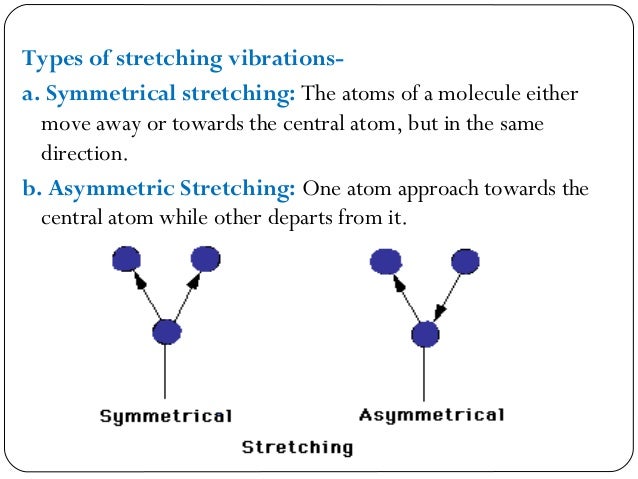
Bending vibrations
- A molecule with three or more atoms can experience a bending vibration, a vibrational mode where the angle between atoms changes
- Change in bond angle
- Occurs at lower energy: 1400-666 cm-1
- There are four types of bend:
- Rocking
- Scissoring
- Wagging
- Twisting
IR active and inactive vibrations
- Not all molecular vibrations absorb IR radiation.
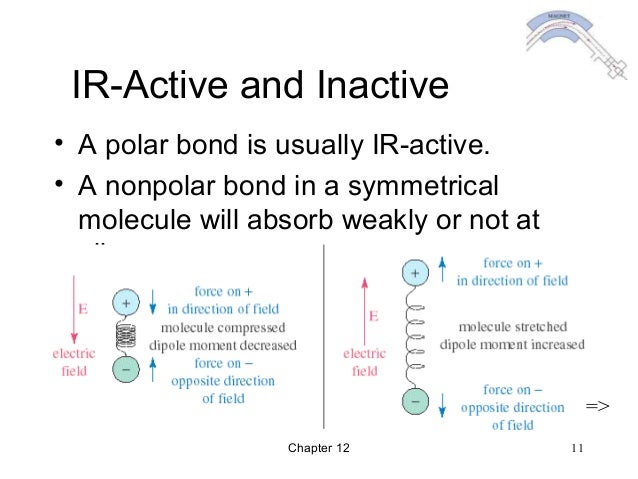
- A polar bond is usually IR-active.
- A nonpolar bond in a symmetrical molecule will absorb weakly or not at all.
- To understand which ones do (and which do not) absorb we need to consider how an electromagnetic field interacts with the chemical bond.
Important points are:
(1) A chemical bond that is polarized has a dipole moment (i.e. charge
separation).
(2) An electric field will interact with the polar bond, causing it either to
stretch or compress.
(3) An electromagnetic wave has a rapidly reversing electric field, so this
radiation rapidly compresses and stretches the polar bond.
(4) If the frequency of the stretching and compression is at the frequency of
the molecule's natural rate of vibration, then energy may be absorbed.
- Vibrations of bonds with dipole moments normally result in the absorption of IR radiation and are said to be IR active.
- If a bond is symmetrical and has zero dipole moment, the electric field does not interact with this bond. If the symmetrical bond is stretched, the dipole moment still remains zero, the vibration produces no change in the dipole moment, there is no absorption of energy. This vibration is said to be IR inactive.
Some general trends about vibration
- Stretching frequencies are higher than corresponding bending frequencies. (It is easier to bend a bond than to stretch or compress it.)
- Bonds to hydrogen have higher stretching frequencies than those to heavier atoms.
- Triple bonds have higher stretching frequencies than corresponding double bonds, which in turn have higher frequencies than single bonds.
Coupled vibrations
In addition to the vibrations mentioned above, the interaction between vibrations can occur (coupling) if the vibrating bonds are joined to a single, central atom. Vibrational coupling is influenced by a number of factors;
- When two bond oscillators share a common atom, they seldom behave as individual oscillators (unless the individual oscillation frequencies are widely different).
- Strong coupling of stretching vibrations occurs when there is a common atom between the two vibrating bonds
- Coupling of bending vibrations occurs when there is a common bond between vibrating groups
- Coupling between a stretching vibration and a bending vibration occurs if the stretching bond is one side of an angle varied by bending vibration
- Coupling is greatest when the coupled groups have approximately equal energies
- No coupling is seen between groups separated by two or more bonds
- The vibrations must be of the same symmetry for coupling
- The interaction is greatest, when the coupled groups absorb (individually) near the same frequency --- the same energies of isolated vibration
- A common bond is required for coupling of bending vibrations.
- Coupling is negligible when groups are separated by one or more carbon atoms and the vibrations are mutually perpendicular.


0 Comments
Thanks for visiting blog. if you have any query please let me know.