In this article, I discuss the ionization technique of mass spectroscopy in detail
Mass spectrometry is based on identifying molecules based on their molecular weight and weights of fragments formed from the molecules as measured by using electric and magnetic fields to measure the masses of ions generated in the gas phase. There are many types of ionization methods are used in mass spectrometry methods.
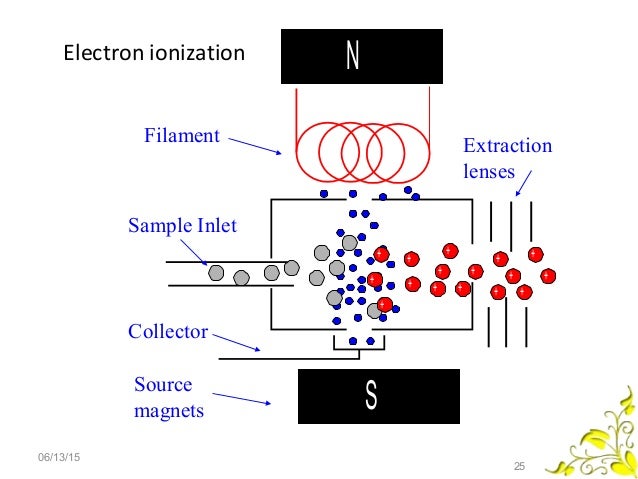
Electron Ionization (EI)
- It is a classical ionization method in mass spectroscopy.
- It is the most widely used and highly developed method.
- It is also known as electron bombardment
- Produces M+. radical cation giving molecular weight.
- It is also known as electron impact ionization.
- A beam of electrons passes through the gas-phase sample. An electron that collides with a neutral analyte molecule can knock off another electron, resulting in a positively charged ion.
- The ionization process can either produce a molecular ion that will have the same molecular weight and elemental composition of the starting analyte, or it can produce a fragment ion which corresponds to a smaller piece of the analyte molecule.
- A heated filament emits electrons which are accelerated by a potential difference of usually 70eV into the sample chamber. Ionization of the sample occurs by removal of an electron from the molecule thus generating a positively charged ion with one unpaired electron.
- The ionization potential is the electron energy that will produce a molecular ion.
- The appearance potential for a given fragment ion is the electron energy that will produce that fragment ion. Most mass spectrometers use electrons with an energy of 70 electron volts (eV) for EI. Decreasing the electron energy can reduce fragmentation, but it also reduces the number of ions formed.
Disadvantages of EI
- It s used only for thermally stable and less volatile sample
- Small amount of sample is ionized
- Unstable molecular ion fragments are formed so readily that are absent from the mass spectrum
- Mass range is Low Typically less than 1,000 Da.
Fast atom bombardment (FAB)
- FAB is a technique that was popular in the '80s to early '90s because it was the first technique that allowed the ionization of non-volatile compounds that could be done simply.
- Used for large compounds with low volatility (eg peptides, proteins, carbohydrates)
- It was done by bombarding a sample in a vacuum with a beam of atoms, typically Ar or Xe accelerated to Kilovolt energies.
- The sample was typically mixed in a matrix. The two most common matrixes were glycerol and 3 Nitro-benzoic acid, thioglycerol, m-nitrobenzyl alcohol, or diethanolamine.
- The ions formed by FAB were adducts to the molecule, where the adducts could be protons, sodium ions, potassium ions, or ammonium ions.
- Gives M+H + or M+Na + ions
- Mass range is Moderate Typically ~300 Da to about 6000 Da.

Limitations
- High chemical background defines detection limits .
- May be difficult to distinguish low-molecular-weight compounds from chemical background .
- Analyte must be soluble in the liquid matrix .
- No good for multiply charged compounds with more than 2 charges
Chemical ionization
- It is Development from EI
- Chemical ionization uses ion-molecule reactions to produce ions from the analyte.
- Softer ionisation technique
- The chemical ionization process begins when a reagent gas such as methane, isobutane, or ammonia is ionized by electron impact.
- Used to produce more abundant molecular ions when the molecule under investigation fragments using EI
- A high reagent gas pressure (or long reaction time) results in ion-molecule reactions between the reagent gas ions and reagent gas neutrals. Some of the products of these ion-molecule reactions can react with the analyte molecules to produce analyte ions.
- Positive CI uses methane, isobutane, or ammonia as reagent gases
- Negative CI uses methane reagent gas in electron capture mode.
- Ionised reagent gas protonate the sample molecules leaving a neutral reagent gas species
- M ass range is Low Typically less than 1,000 Da.
Limitations
- Sample must be thermally volatile and stable .
- Less fragmentation than EI, fragment pattern not informative or reproducible enough for library search .
- Results depend on reagent gas type, reagent gas pressure or reaction time, and nature of sample.
Electrospray ionization (ESI)
- ESI is the ionization technique that has become the most popular ionization technique
- Electrospray also known as : – Ionspray, Nanospray, Sonic Spray, “Pure” Electrospray – ESI, ES, IS.
- Softest ionization technique
- The electrospray is created by putting a high voltage on a flow of liquid at atmospheric pressure, sometimes this is assisted by a concurrent flow of gas. The created spray is directed to an opening in the vacuum system of the mass spectrometer, where the droplets are de-solvated by a combination of heat, vacuum, and acceleration into a gas by voltages.
- Best for polar non-volatile compounds (proteins, peptides, nucleic acids, Pharmaceuticals, natural products)
- The sample solution is sprayed across a high potential difference (a few kilovolts) from a needle into an orifice in the interface. Heat and gas flows are used to desolvate the ions existing in the sample solution.
- Electrospray ionization can produce multiply charged ions with the number of charges tending to increase as the molecular weight increases.
- Not very tolerant of non-volatile salts
- Ions are ejected from charged vapour droplets to gas phase producing M+H+ or M - H- ion.
Limitations
- Multiply charged species require interpretation and mathematical transformation (can sometimes be difficult) .
- Complementary to APCI. No good for uncharged, non-basic, low-polarity compounds (e.g.steroids) . Very sensitive to contaminants such as alkali metals or basic compounds .
- Relatively low ion currents .
- Relatively complex hardware compared to other ion sources
Another method will be discussed in another article


0 Comments
Thanks for visiting blog. if you have any query please let me know.