THIS ARTICLE DISCUSSES THE INSTRUMENTATION OF UV SPECTROSCOPY IN DETAIL.
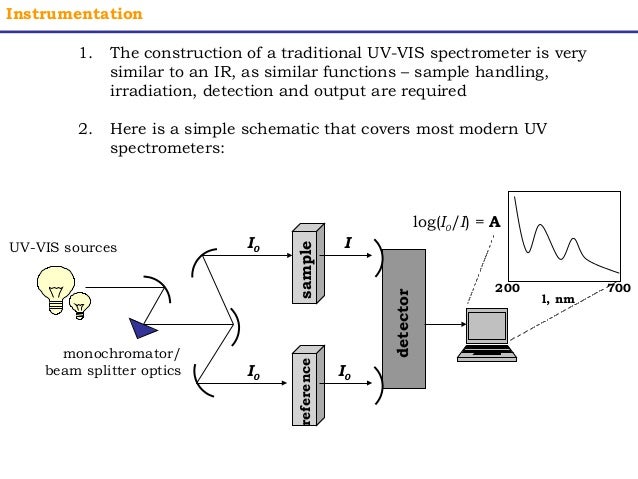
A double beam ultraviolet/visible spectrophotometer is commonly used to measure the amount of radiation absorbed by the sample at each wavelength of the ultraviolet and visible region. The typical ultraviolet-visible spectrophotometer consists of a
- Sources (UV and visible)
- Wavelength selector (monochromator)
- Beam splitter
- Sample containers
- Detector
- Signal processor and readout
Source
It is important that the power of the radiation source does not change abruptly over its wavelength range. The light source is usually a deuterium lamp or hydrogen discharge lamp, which emits electromagnetic radiation in the ultraviolet region (200-400 nm) of the spectrum. A second light source, a tungsten lamp, is used for wavelengths in the visible region (400-800) of the spectrum.
Wavelength selector (monochromator)
The radiation beam is passed through a monochromator (a prism or a diffraction grating) where it is dispersed into its components wavelengths. A system of slits focuses the desired wavelength on the sample cell. All monochromators contain the following component parts;
- An entrance slit
- A collimating lens
- A dispensing device (usually a prism or a grating)
- A focusing lens
- An exit
Sample and reference cell.
The monochromater radiation is spilled into two beams of equal intensity.One beam passes through the sample and the other beam serves as a reference. The containers for the sample and reference solution must be transparent to the radiation which will pass through them.For measurements in the ultraviolet region of the spectrum, however, glass and plastic cannot be used because they absorb ultraviolet radiation. Instead, cells made of quartz must be used since quartz does not absorb radiation in this region.Quartz or fused silica cuvettes are required for spectroscopy in the UV region. These cells are also transparent in the visible region.
Detector
If the sample absorbs the radiation ar a particular wavelength, the intensity of asample beam will decrease. The light that passes through the sample cell reaches the detector, which records the intensity of the transmitted light I. The detector is generally a photomultiplier tube, although in modern instruments photodiodes are also used. The dectector will make a comparison of the intensities of the two beams at each wavelength.It consists of a photoemissive cathode (a cathode that emits electrons when struck by photons of radiation), several dynodes (which emit several electrons for each electron striking them), and an anode.
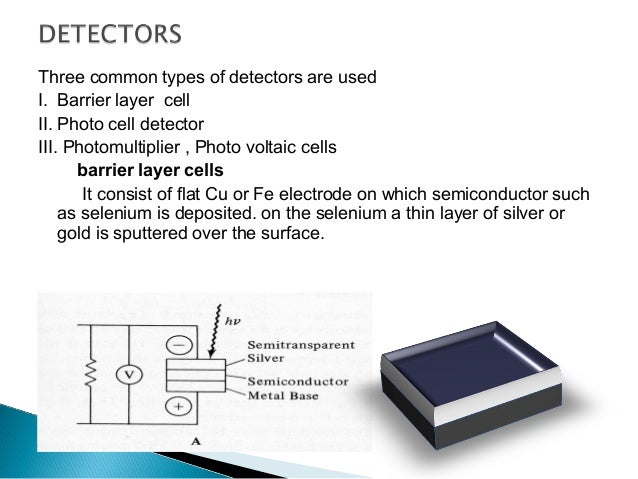
Recorder
This comparison is recrorded by the recorder in form of graph which is aplot of absorbance against wavelength over the entire region of study. Such a graph is known as spectrum.
A modern improvement on the traditional spectrophotometer is the diode-array spectrophotometer. A diode array consists of a series of photodiode detectors positioned side by side on a silicon crystal. Each diode is designed to record a narrow band of the spectrum. The diodes are connected so that the entire spectrum is recorded at once. This type of detector has no moving parts and can record spectra very quickly. Furthermore, its output can be passed to a computer, which can process the information and provide a variety of useful output formats. Since the number of photodiodes is limited, the speed and convenience described here are obtained at some small cost in resolution. For many applications, however, the advantages of this type of instrument outweigh the loss of resolution.
For UV/VIS spectral study, the sample is usually used in the form of a very dilute solution in asolvent that is transparent ( does not absorb) in the region to be studied. The sample solution is usually taken in a quartz cell which ius placed in the path of a sample beam.






0 Comments
Thanks for visiting blog. if you have any query please let me know.