- The Purcell group at Harvard University and the Bloch group at Stanford University independently developed NMR spectroscopy in the late 1940s and early 1950s. Edward Mills Purcell and Felix Bloch shared the 1952 Nobel Prize in Physics for their discoveries.
- Nuclear magnetic resonance (NMR) is a spectroscopic method that is even more important to the organic chemist than infrared spectroscopy. Many nuclei may be studied by NMR techniques, but hydrogen and carbon are most commonly available. Whereas infrared (IR) spectroscopy reveals the types of functional groups present in a molecule, NMR gives information about the number of magnetically distinct atoms of the type being studied.
- Nuclear magnetic resonance spectroscopy', most commonly known as NMR spectroscopy, is a research technique that exploits the magnetic properties of certain atomic nuclei.
- This type of spectroscopy determines the physical and chemical properties of atoms or the molecule in which they are contained.
- Nuclear Magnetic Resonance (NMR) spectroscopy is an analytical chemistry technique used in quality control and research for determining the content and purity of a sample as well as its molecular structure.. For example, NMR can quantitatively analyze mixtures containing known compounds.
- The combination of IR and NMR data is often sufficient to determine completely the structure of an unknown molecule.
- It can provide detailed information about the structure, dynamics, reaction state, and chemical environment of molecules.
- It is also increasingly used in inorganic chemistry and biochemistry, where it also provides a lot of valuable structural information.
- Nuclear Magnetic Resonance is a property of the nucleus of an atom, concerned with what is known as nuclear spin (I)
- Once the basic structure is known, NMR can be used to determine molecular conformation in solution as well as studying physical properties at the molecular level such as conformational exchange, phase changes, solubility, and diffusion.
Principle of NMR
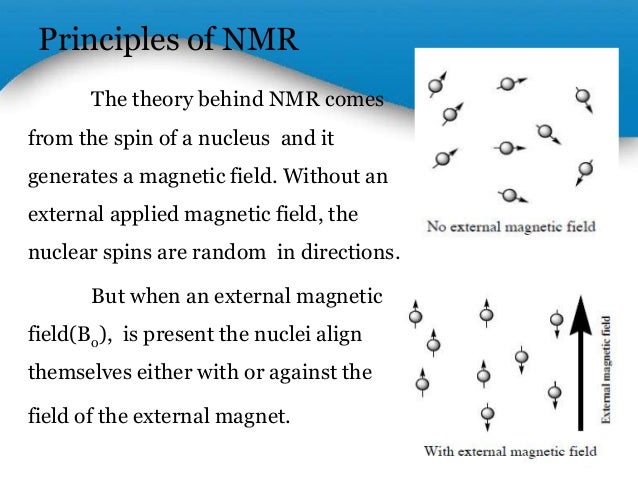
- The principle behind NMR is that many nuclei have spin and all nuclei are electrically charged.In fact, any atomic nucleus that possesses either odd mass, odd atomic number, or both has a quantized spin angular momentum and a magnetic moment.
- Subatomic particles (electrons, protons and neutrons) can be imagined as spinning on their axes. In many atoms (such as 12C) these spins are paired against each other, such that the nucleus of the atom has no overall spin. However, in some atoms (such as 1H and 13C) the nucleus does possess an overall spin.
- The rules for determining the net spin of a nucleus are as follows;
- If the number of neutrons and the number of protons are both even, then the nucleus has NO spin.
- If the number of neutrons plus the number of protons is odd, then the nucleus has a half-integer spin (i.e. 1/2, 3/2, 5/2)
- If the number of neutrons and the number of protons are both odd, then the nucleus has an integer spin (i.e. 1, 2, 3).
NUCLEAR MAGNETIC MOMENTS
- The overall spin, I, is important. Quantum mechanics tells us that a nucleus of spin I will have 2I + 1 possible orientations. A nucleus with spin 1/2 will have 2 possible orientations. In the absence of an external magnetic field, these orientations are of equal energy.
- When a nucleus with I = 1/2 is placed in a magnetic field, it can either align itself with the field (lower energy) or against it (higher energy). If radio waves are applied, nuclei in the lower energy state can absorb the energy and jump to the higher energy state.We can observe either the absorption of energy, or the subsequent release of energy as the nucleus "relaxes" back to the lower energy state.
Spin states are not of equivalent energy in an applied magnetic field because the nucleus is a charged particle, and any moving charge generates a magnetic field of its own. Thus, the nucleus has a magnetic moment generated by its charge and spin. A hydrogen nucleus may have a clockwise (+1/2 ) or counterclockwise (⎯ 1/ 2 ) spin, and the nuclear magnetic moments (m) in the two cases are pointed in opposite directions. If an external magnetic field is applied, an energy transfer is possible between the base energy to a higher energy level .The energy transfer takes place at a wavelength that corresponds to radio frequencies and when the spin returns to its base level, energy is emitted at the same frequency.The signal that matches this transfer is measured in many ways and processed in order to yield an NMR spectrum for the nucleus concerned.






0 Comments
Thanks for visiting blog. if you have any query please let me know.