- Infrared light was discovered in 1800 by Sir Frederick William Herschel. Herschel, born in Hanover, Germany, decided to investigate the temperature of each color of the spectrum produced when sunlight is passed through a prism.
| Sir Frederick William Herschel |
- Infrared (IR) spectroscopy is one of the most common and widely used spectroscopic techniques employed mainly by inorganic and organic chemists due to its usefulness in determining structures of compounds and identifying them
- Infrared Spectroscopy is the analysis of infrared light interacting with a molecule.
- Infrared (IR) Spectroscopy uses a beam of infrared light to analyze the structure of organic compounds.
- Almost any compound having covalent bonds, whether organic or inorganic, absorbs various frequencies of electromagnetic radiation in the infrared region of the electromagnetic spectrum.
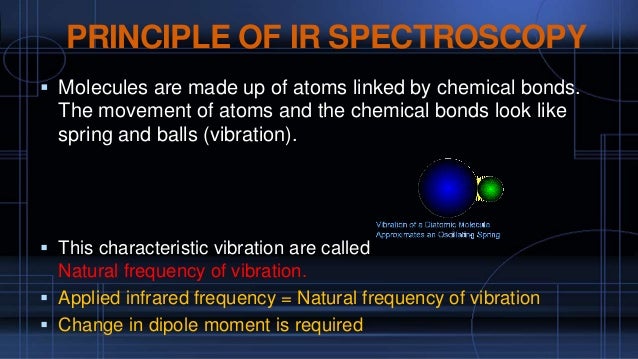
- Absorption of IR radiations causes vibrational transitions of molecule so IR spectroscopy is also known as vibrational spectroscopy.
- This region lies at wavelengths longer than those associated with visible light, which range from approximately 400 to 800 nm (1 nm =10−9 m), but lies at wavelengths shorter than those associated with microwaves, which are longer than 1 mm.
- The term "infra red" covers the range of the electromagnetic spectrum between 0.78 and 1000 mm. In the context of infra red spectroscopy, wavelength is measured in "wavenumbers", which have the units cm-1.
- This can be analyzed in three ways by measuring absorption, emission and reflection.
- Radiations in this region can be utilized in organic structure determination by making use of the fact that it is absorbed by interatomic bonds in organic compounds.
- It is used by chemists to determine functional groups in molecules.IR Spectroscopy measures the vibrations of atoms, and based on this it is possible to determine the functional groups.
- IR radiation does not have enough energy to induce electronic transitions as seen with UV. Absorption of IR is restricted to compounds with small energy differences in the possible vibrational and rotational states.IR radiation does not have enough energy to induce electronic transitions as seen with UV. Absorption of IR is restricted to compounds with small energy differences in the possible vibrational and rotational states.
- If the frequency of the radiation matches the vibrational frequency of the molecule then radiation will be absorbed, causing a change in the amplitude of molecular vibration.
- An IR spectrum usually extends from radiation around 4000 cm-1 to 600 cm-1 and can be split into the functional group region and the fingerprint region.
Functional group and finger print region
The fingerprint region is the region to the right-hand side of the diagram (from about 1500 to 500 cm-1) usually contains a very complicated series of absorptions. These are mainly due to all manner of bending vibrations within the molecule.The fingerprint region is different for each
molecule just like a fingerprint is different for each person.Two different molecules may
have similar functional group regions because they have similar functional groups, but
they will always have a different fingerprint region. Additionally, since stretching vibrations are typically found in
the functional group region, they are the most convenient vibrations to analyze when
determining the types of bonds a molecule has.Finger print region is related to bending vibrations and functional group is related to stretching vibrations.
- It is useful to divide the infra red region into three sections; near, mid and far infra red;
| Region | Wavelength range (mm) | Wavenumber range (cm-1) |
| Near | 0.78 - 2.5 | 12800 - 4000 |
| Middle | 2.5 - 50 | 4000 - 200 |
| Far | 50 -1000 | 200 - 10 |
The most useful I.R. region lies between 4000 - 670cm-1.
- Most chemists refer to the radiation in the vibrational infrared region of the electromagnetic spectrum in terms of a unit called a wavenumber (n ), rather than wavelength (m or mm).The main reason chemists prefer to use wavenumbers as units is that they are directly proportional to energy (a higher wavenumber corresponds to a higher energy).Thus, in terms of wavenumbers, the vibrational infrared extends from about 4000 to 400 cm−1..
- Infrared radiation is a form of electromagnetic radiation of slightly lower energy (longer wavelength) than visible light. When this electromagnetic wave interacts with a covalent bond which has an electrical dipole, the energy is absorbed and the bond will start oscillating back and forth. Any such vibration that causes a change in the dipole of the molecule should absorb IR radiation.
- A single atom cannot absorb IR radiation, as it has no bonds. For a symmetric molecule such as hydrogen (H-H), there is no change in dipole when the molecule vibrates (stretches and contracts), so again it will not absorb IR. But for an asymmetric diatomic molecule such as H-F, the dipole moment will change as the bond stretches or contracts, so HF does absorb IR radiation.
- It is invisible to human eyes, but people can feel it as heat.
- IR radiation is one of the three ways heat is transferred from one place to another, the other two being convection and conduction.









0 Comments
Thanks for visiting blog. if you have any query please let me know.