IN THIS ARTICLE I DISCUSS ABOUT THE APPLICATION OF IR SPECTROSCOPY

- Infrared radiation lies between the visible and microwave portions of the electromagnetic spectrum.
- Infrared waves have wavelengths longer than visible and shorter than microwaves, and have frequencies which are lower than visible and higher than microwaves.
- If matter is exposed to electromagnetic radiation, e.g. infrared light, the radiation can be absorbed, transmitted, reflected, scattered or undergo photoluminescence.
- The primary source of infrared radiation is thermal radiation. (heat).
- A molecule can be characterized (identified) by its molecular vibrations, based on the absorption and intensity of specific infrared wavelengths.
There are many applications of IR spectroscopy.
1. Analysis of inorganic complexes.
This technique is used to study the geomatrical isomerisom of inorganic complexes.
eg. Cis and trans isomer of Bipyridyl cobalt tricholoride
Character cis-isomer trans-isomer
colour pink violet
symmetry low relatively high
Itensity of bands greater lesser
2. Quantitative analysis
- Quantitative infrared absorption methods differ somewhat from UV/VIS molecular spectroscopic methods because of the greater complexity of the spectra, the narrowness of the absorption bands, and the instrumental limitations of infrared instruments.Quantitative data obtained with dispersive infrared instruments are generally poorer in quality to data obtained with UV/VIS spectrophotometers.
- This technique is used to determine the concentration of one of the functional group of compounds to be estimated. eg. mixture of hexane and hexanol
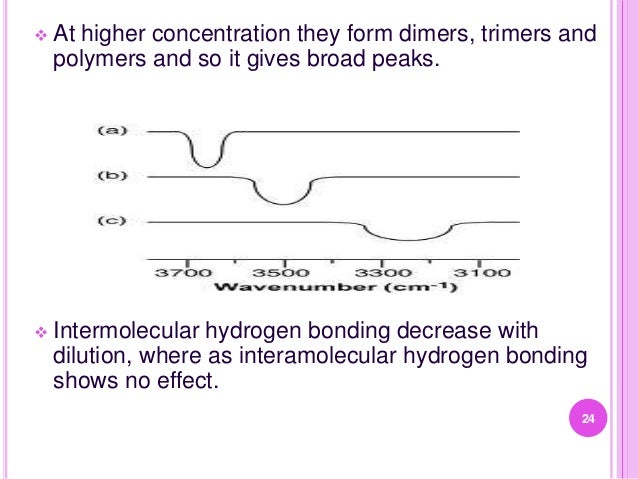
S 3. Study of Hydrogen bonding


4
4 .Biological Applications
- Infrared spectroscopy has proved to be a powerful tool for the study of biological molecules and the application of this technique to biological problems is continually expanding, particularly with the advent of increasingly sophisticated sampling techniques such as infrared imaging.
- Biological systems have all been successfully studied by using infrared spectroscopy
- Infrared spectroscopy can provide valuable structural information about lipids, which are important molecular components of membranes.
- The infrared spectra of proteins exhibit absorption bands associated with their characteristic amide group.Infrared spectroscopy has been used in a large number of studies of proteins in a range of environments.
- The nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), may be studied by using infrared spectroscopy . The spectra of nucleic acids may be divided into the modes due to the constituent base, sugar and phosphate groups.
- FTIR spectroscopy may be used as a tool for the investigation of plant material is in the examination of the quality of an animal food such as alfalfa.
5.Application in pharmaceutical industry
Infrared spectroscopy has been extensively used in both qualitative and quantitative
pharmaceutical analysis [1–3]. This technique is important for the evaluation
of the raw materials used in production, the active ingredients and the excipients
(the inert ingredients in a drug formulation, e.g. lactose powder, hydroxypropyl
cellulose capsules, etc.).
- Detection of impurities can be done.
- Quality control tool: it helps in determining the composition and amount of product in sample
- Identification of sample in research center.
- Flavouring agent and colouring agent can also be identified by using this technique
6.Qualitative analysis
- Used for identifying organic, inorganic, and biological species.
- The time required to perform a structural determination was reduced by a factor of ten, one hundred, or even one thousand (by FTIR modern instruments).
- Identification of an organic compound is a two-step process.
- The first step involves determining what functional groups are most likely present by examining the group frequency region.
- The second step then involves a detailed comparison of the spectrum of the unknown with the spectra of pure compounds that contain all of the functional groups found in the first step.
- The fingerprint region, from 1200 to 600 cm -1 is particularly useful because small differences in the structure and constitution of a molecule result in significant changes in the appearance and distribution of absorption peaks in this region
7. 7.Study of conformational analysis
- Tis technique is used to study abut the relative stability of various conformational of cyclic compounds.eg in cyclohexane both boat and chair form exist
- As per IR selection rules there 18 C-C and CH2 rocking and twisting is possible for boat form.
- As for IR selection ther are five band for chair form.
8. 8. Analysis of organic compounds
- Identification or organic compound is carried out by using this technique.
- Rates of organic reaction are also studied.
- Determinination of purity of sample.
- Identification of isomer like functional isomer, geomatric isomer,tautomer etc.
- Identification of functional group and elucidation of structure of any compound can also be done.
9.Disease diagnose
Infrared spectroscopy has emerged as a powerful tool for characterizing
tissue and disease diagnosis.The pressure-dependence
on parameters such as wavenumber, intensity and bandshape may provide further
information about the structural changes associated with malignancy. For
example, it is possible to detect cervical cancer arising from a pre-malignant
state termed dysplasia by using infrared band differences among normal, dysplastic
and malignant cervical cells.Infrared mapping is emerging as a widely used approach for the study of diseased
tissue. The identification of diseased regions of tissues is
complex and the use of multivariate pattern recognition methods is appropriate
10. Computer search system
- Virtually all infrared instrument manufactures now offer computer search systems to assist chemist in identifying compounds from stored infrared spectral data.
- The position and relative magnitudes of peaks in the spectrum of the analyte are determined and stored in memory to give a peak profile, which can then be compared with profiles of pure compounds stored.
- The computer then matches profiles and prints a list of compounds having spectra similar to that of the analyte.
11.Use in Clinical Chemistry
Common infrared tests in clinical chemistry include glucose, blood and urine
analyses.The use of near-infrared spectroscopy and attenuated
total reflectance (ATR) spectroscopy in the mid-infrared region lend themselves
to the study of glucose in such aqueous environments, and partial least-squares
(PLS) and principal component analysis (PCA) methods have been successfully
applied to quantitative analysis of glucose samples
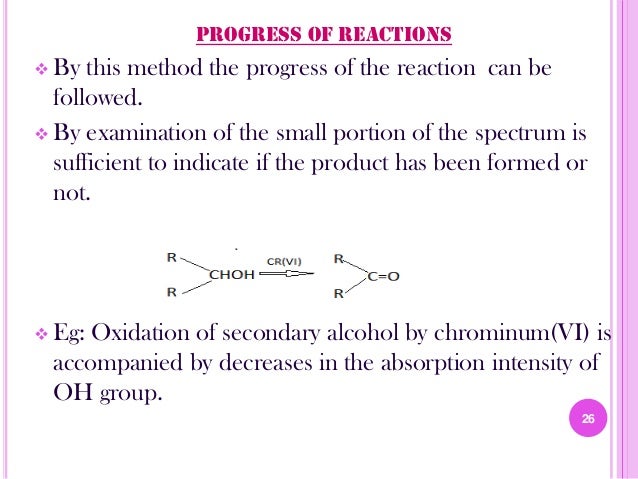
12. Study of progrss of reaction

13. 13. Food science
Both mid-infrared and near-infrared techniques may be used to obtain qualitative
and quantitative information about food samples . Foods are complex
mixtures, with the main components being water, proteins, fats and carbohydrates.Infrared spectroscopy has long been employed to study fats and oils . One
important infrared method is the determination of trans-isomers in fats and oils
(which mainly consist of triglycerides). Near-infrared (NIR) spectroscopy is widely used in the food industry as a
fast routine analytical method for the quantitative measurement of water, proteins,
fats and carbohydrates . NIR spectroscopy has been used for many years in the dairy industry to examine
milk.
14.Capabilities of Infrared Analysis
- Identification and quantitation of organic solid, liquid or gas samples.
- Analysis of powders, solids, gels, emulsions, pastes, pure liquids and solutions, polymers, pure and mixed gases.
- Infrared used for research, methods development, quality control and quality assurance applications.
- Samples range in size from single fibers only 20 microns in length to atmospheric pollution studies involving large area.
15.Detection of impurity
Impurity can be identified by the comparison of test and standard spectrum. Pure spectrum consist of sharp peaks and impure consist of bent one and alsp some additional peaks.
16. Shape of symmetry of molecule
- Nitrogen dioxide IF. linear..........only 2 band are present. IF bent..... three band shoud be present. Actual spectrum shows three peaks at 750, 1323 and 1616 cm-1
17. Agricultural Applications
Commercial grains are commonly analysed by using NIR spectroscopy .
The major constituents of grains are water, protein, oil, fibre, minerals and carbohydrates
and it is commercially important to quantitate the composition. The
NIR spectra of such materials are, thus, dominated by the overtones and combination
bands of C–H, N–H, O–H and C=O bonds.
18.Presence of water in sample
If lattice water is present in a sample, spectra will contain three characterstics bands at 3600-3200cm-1 and 600-300cm-1
19.Recent application of IR spectroscopy
- Forensic application
- Hair imaging
- Pharmaceutical research
- Polymer analysis
- Geometrical isomerism
- Quality assurance and control
- Study of keto-enol tautomerism
- Combustion and fire detection
- Atmosphere and environment study
- Measurement of paints and varnishes
- Assesment of brain function
- Examination of old paintings
- Measuring electric and magnetic fields
20. Pulp and Paper Industries
Infrared spectroscopy plays an important role in quality control in the pulp and
paper industries . Paper is comprised of cellulose fibres, inorganic fillers
and binders. In the mid-infrared region, additives, such as polymers and calcium
carbonate, may be identified in paper. The majority of bands in a paper spectrum
are due to cellulose. The latter shows an O–H stretching band near 3330 cm−1,
C–H stretching bands in the 3000–2900 cm−1 region, C–H bending bands in the
range 1500–1300 cm−1, and a C–O ether stretching band at 1030 cm−1.
21 21. Environmental Applications
Infrared spectroscopy has been applied to a broad range of environmental sampling
problems, including air, water and soil analysis . The development
of remote sensing infrared techniques has been particularly useful in this field
and simple practical infrared techniques have been developed for measuring
trace gases and atmospheric compositions
22. Determination of molecular structure
With the help of other spectroscopic techniques it is used for the determination of molecular structure of any compound.Identification is done based on the position of absorption band on the spectrum.Absence of band of particular group indicate absence of that group in the compound.
23 .Some others applications of IR
- Study of isomerism in organic chemistry
- Study of purity of sample
- Identification of functional group
- Analysis of petroleum, HCs ,Oils and grease content
- Quantitative analysis of multi component mixture of oxygen-sulphur anion by ATR spectroscopy
- Characterization of heterogenous catalysts by diffuse Reflectance spectroscopy
- Identify odour and taste component of food
- Determine atmospheric pollution from atmosphere.
- Mid -IR used for organic,inorganic and biological sudies
- Far-IR treatment of chronic health and observe intestellar gases


0 Comments
Thanks for visiting blog. if you have any query please let me know.