In this article i disscus about the applications or importance or significance of UV in chemistry and other world
UV spectroscopy is basically is important tool i n chemistry.It is also known as electronic spectroscopy because their is promotion of electron from ground stat to exited stat occur. There are many application of UV spectroscopy.
- In research, ultraviolet/visible spectroscopy is used more extensively in assaying than in identification.
- UV spectroscopy is used to study about the electronic transitions between rotational and electronic level.
- The word spectroscopy shows that we will use electromagnetic spectrum to gain information about organic as well as inorganic molecule.
4. Detection of impurities.
UV spectroscopy is one of the best method to identify the impurities in organic molecule.Additional peaks can be observed in the spectrum due to impurities in the sample and it can be compared with that of standard raw material.By also measuring the absorbance at specific wavelength,the impurities cab be detected.
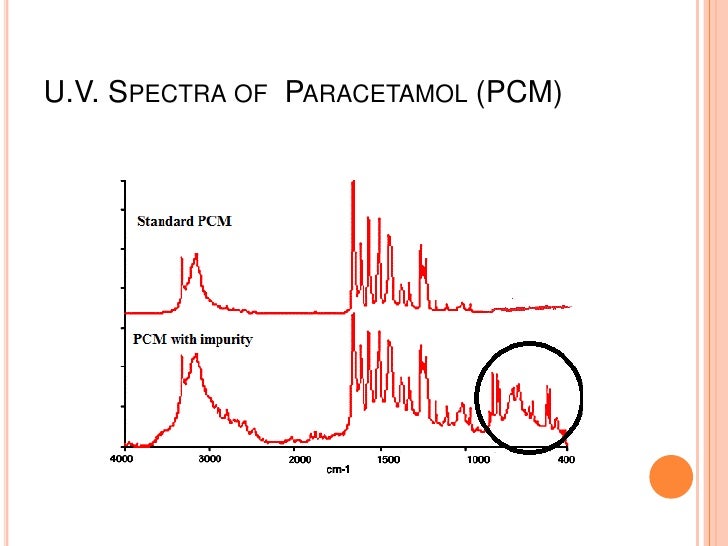
5. Identification of an unknown compound
We can easily identify unknown compounds by using UV spectroscopy.First of all we take the spectrum of unknown compound and compare it with the reference spectrum.If both the spectrum are resemble with each other then we easily identified that unknown compound.
6. Structure elucidation of organic compound
Structure elucidation of organic compounds can easily be done with the help of UV spectroscopy .We easily identify the presence and absence of unsaturation and presence of hetero atom in a compund by using this.From the location of peaks and combination of peaks, it can be concluded that whether the compound is saturated or unsaturated,hetero atom is present or not etc.
7. Detection of functional group
UV spectroscopy is used to detect the presence or absence of chromophore in the compound. But we cannot use this technique for the identification of chromophore in complex molecules. If the spectrum of a compound comes out to be transparent above 200 nm than it confirms the absence of –
a) Conjugation b) A carbonyl group c) Benzene or aromatic compound d) Bromo or iodo
atoms.
8.Quantitative analysis
Quantitative study of those compounds that absorb UV radiations can also be done with the help of UV. This determination is based on Beer's Law. which is as follow
ABSORBANCE = log(1/T) = -log(T) = εdC
Where:
ε = Molar absorptivity (L mol-1 cm-1)
d = Path length of the cuvette containing the sample (cm)
C = Concentration of the compound in the solution (mol L-1)
9.Detection of extent of conjugation
The extent of conjugation in the polyenes can be detected with the help of UV spectroscopy. With the increase in double bonds the absorption shifts towards the longer wavelength. If the double bond is increased by 8 in the polyenes then that polyene appears visible to the human eye as the absorption comes in the visible region.
10. Qualitative study
UV can also characterize those types of compound which absorb UV radiations. Identification is done by comparing the absorption spectrum with the spectra of known compounds.
11.Determination of configurations of geometrical isomers
It is observed that cis-alkenes absorb at different wavelength than the trans-alkenes. The two isomers can be distinguished with each other when one of the isomers has non-coplanar structure due to steric hindrances. The cis-isomer suffers distortion and absorbs at lower wavelength as compared to trans-isomer.
12. Chemical kinetics
Kinetics of chemical reaction can also be studied with the help of UV.The UV radiations are passed through the reaction cell and the absorbance change can be observed.
13. As a HPLC detector
A uv/vis spectrophotometer can be used as detector as HPLC
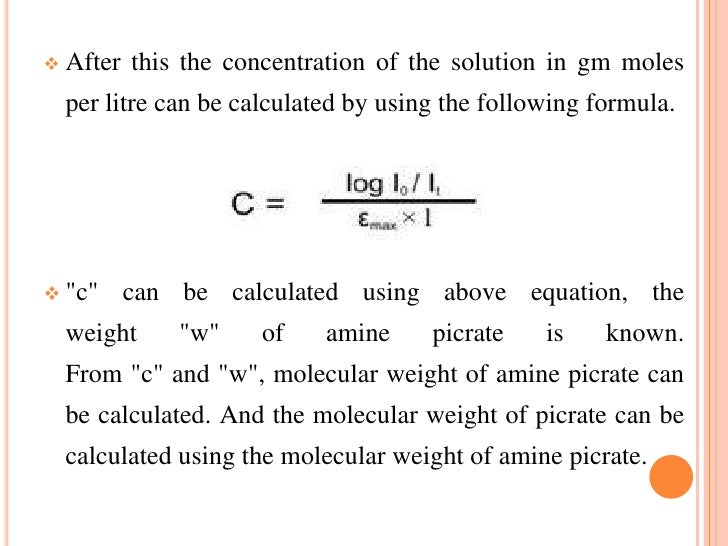
14. Molecular weight determination
Molecular weight of compound can be measured spectrophotometrically by preparing the suitable derivative of these compounds.for example
If we want to measure the molecular weight of amine then it is converted to amine picrate .Then known concentration of amine picrate is dissolved in a litre of solution and its optical density is measured in lemda mex 380 nm..
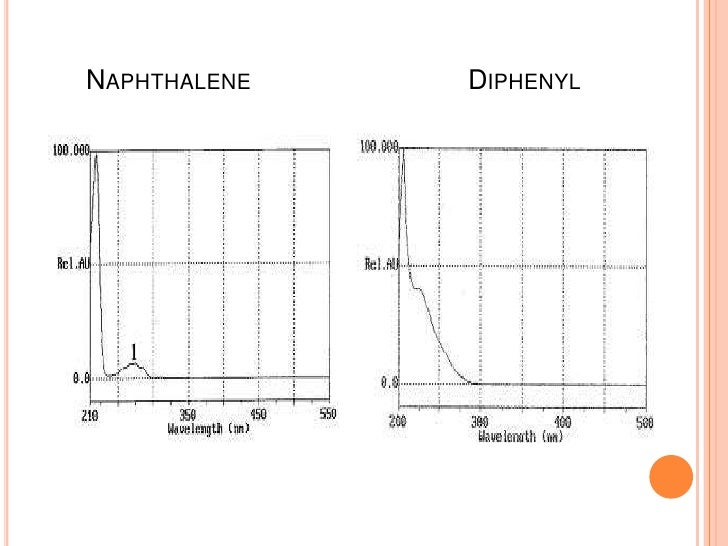
15.Examination of polynuclear structure of hydrocarbons
Benzene and polynuclear hydrocarbons have charactersictics spectra in ultaviolet and visible region.Thus identification of polynuclear hydrocarbons can be made by comparison with the spectra of known polynuclear hydrocarbons.


0 Comments
Thanks for visiting blog. if you have any query please let me know.